


The term 'change of phase' means the same thing as the term 'change of state'.
There are four states, or phases, of matter:
- Solid
- Liquid
- Gas
- Plasma
We will not be discussing the plasma state here.
When a substance changes from one state, or phase, of matter to another we say that it has undergone a change of state, or we say that it has undergone a change of phase. For example, ice melts and becomes water; water evaporates and becomes water vapor.
These changes of phase always occur with a change of heat. Heat, which is energy, either comes into the material during a change of phase or heat comes out of the material during this change. However, although the heat content of the material changes, the temperature does not.
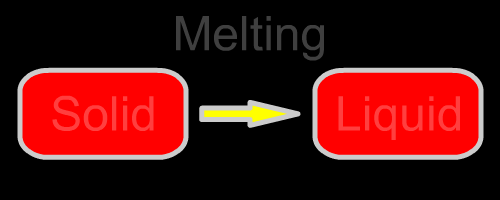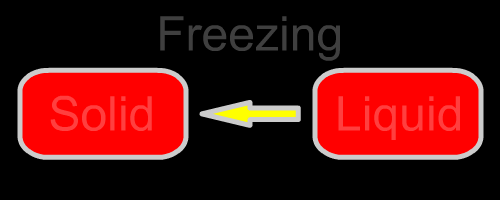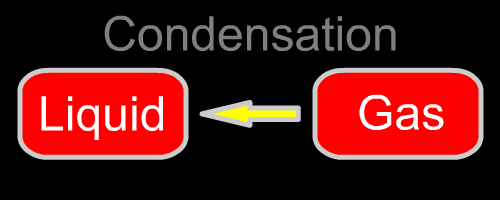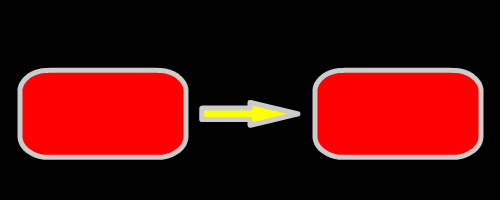
Here are the five changes of phase. They are diagrammed in the opening animation and listed below.
| Description of Phase Change | Term for Phase Change | Heat Movement During Phase Change | Temperature Change During Phase Change |
| Solid to liquid | Melting | Heat goes into the solid as it melts. | None |
| Liquid to solid | Freezing | Heat leaves the liquid as it freezes. | None |
| Liquid to gas | Vaporization, which includes boiling and evaporation | Heat goes into the liquid as it vaporizes. | None |
| Gas to liquid | Condensation | Heat leaves the gas as it condenses. | None |
| Solid to gas | Sublimation | Heat goes into the solid as it sublimates. | None |
So, how could there be a change in heat during a state change without a change in temperature? During a change in state the heat energy is used to change the bonding between the molecules. In the case of melting, added energy is used to break the bonds between the molecules. In the case of freezing, energy is subtracted as the molecules bond to one another. These energy exchanges are not changes in kinetic energy. They are changes in bonding energy between the molecules.
If heat is coming into a substance during a phase change, then this energy is used to break the bonds between the molecules of the substance. The example we will use here is ice melting into water. Immediately after the molecular bonds in the ice are broken the molecules are moving (vibrating) at the same average speed as before, so their average kinetic energy remains the same, and, thus, their Kelvin temperature remains the same.
Below is a picture of solid ice melting into liquid water. The molecule of ice and the molecule of water (the black balls) are moving with the same rate of vibration in this diagram. This is meant to show that they have the same average speed and thus the same average kinetic energy (since they have the same mass) and thus the same Kelvin temperature. The motions are, though, greatly exaggerated. Actually, the motions of the molecules should be considered tiny vibrations.



|
At the moment of melting the average kinetic energy of the
molecules does not change. The heat is used to break the bonds between the ice molecules as they turn into a liquid phase. Since the average kinetic energy of the molecules does not change at the moment of melting, the temperature of the molecules does not change. Since both the ice and the water molecules have the same average kinetic energy at the time of melting, the temperatures of both are the same. |
In the ice the molecules are strongly bonded to one another, thus forming a rigid solid. When heat is added to the ice these bonds are broken and the ice melts. The molecules afterward bond to one another with less strength and a different geometry, and water is formed.
Now, before the melting, the molecules were actually moving when in the solid state. They were vibrating back and forth. They had an average kinetic energy. So they had a Kelvin temperature proportional to this average kinetic energy.
After the melting the water molecules are still vibrating. And they have the same average kinetic energy as they had before the melting. So, the water is at the same temperature at the moment after the melting that the ice was at the moment before the melting.
Heat came into the situation, but it was not used to change the kinetic energy of the molecules. It was used to change the bonding between the molecules. Breaking the bonds between the molecules of the ice requires energy, and this energy is the added heat.
In a similar way heat enters a liquid to change the molecular bonding when the liquid boils or evaporates into a gas, and heat enters a solid to change the molecular bonding when it sublimates into a gas.
In an inverse way heat leaves a gas to change the molecular bonding when the gas condenses into a liquid, and heat leaves a liquid to change the molecular bonding when it freezes into a solid.
In none of these changes of state is the heat (energy) that is input or output used to change the speed of the molecules. The average speed of the molecules is the same before and after a phase change, and so is the average kinetic energy.
| Heat (energy) is transferred into the ice. |  |
The heat is used to break the bonds between molecules, not to increase the average kinetic energy of the molecules. |  |
Since the bonds among the ice molecules have been broken, water is formed. The water molecules, at this moment, have the same average kinetic energy as they did when they were ice. |  |
Since the ice and water molecules both have the same average kinetic energy, they are at the same Kelvin temperature. |