



Definition of Fission
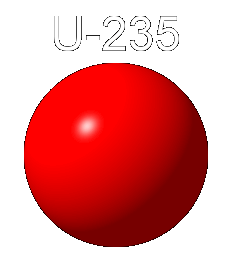
Fission is the process through which large nuclei break apart into smaller nuclei and a few neutrons. An isotope of uranium, U-235, will undergo fission. If struck by a neutron, the nucleus of U-235 will become unstable and break into two other smaller nuclei and a few neutrons. This process releases energy.
U-235
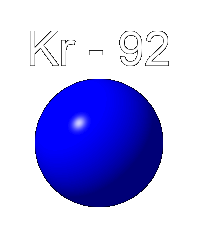
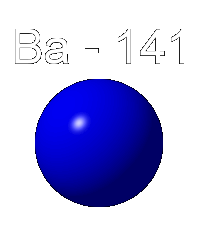
Below we see an animation in which a nucleus of uranium 235 absorbs a neutron. This raises the nucleus to a higher energy state in which the separate protons and neutrons move about more vigorously, thus making the nucleus unstable. Uranium 235 breaks into two smaller nuclei, here shown as Beryllium 141 and Krypton 92, although other products are possible. Also emitted are a few neutrons. In this animation three neutrons are released.







The above animation is only a simple illustration. The animation does not simulate the conservation of momentum or represent the relative speeds of the particles, for example.
E = mc2
When fission occurs, the products have less mass than the reactants. That is, in the above reaction one atom of Beryllium 141, one atom of Krypton 92, and three neutrons have less mass than one atom of Uranium 235 and one neutron. This loss of mass is realized as an increase in energy according to Einstein's equation E = mc2.